For adults with moderately to severely active ulcerative colitis who are steroid dependent or have failed or were intolerant to conventional therapy.
Take a fresh approach to UC with SIMPONI®
SIMPONI® is a prescription medicine and the first and only injectable UC treatment approved by the FDA to both control symptoms and begin to improve the appearance of the intestinal lining for moderately to severely active adult UC patients who have not responded well to certain other UC medications or find it necessary to continue taking steroid medications.
Ulcerative Colitis Treatment With SIMPONI®
While individual results may vary, SIMPONI® was proven in clinical studies to:
- Help people with moderately to severely active UC get their symptoms under control in as few as 6 weeks
- Begin to improve the appearance of the intestinal lining in as few as 6 weeks
- Keep symptoms under control with a single injection just once every 4 weeks for those people who responded to SIMPONI®
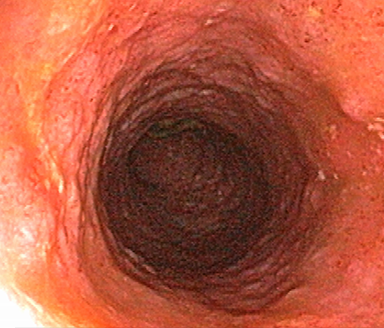
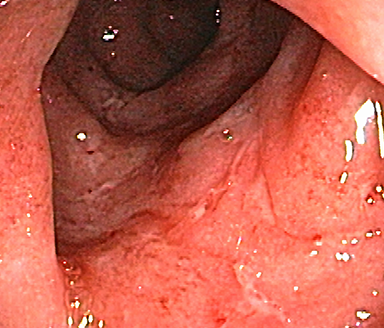
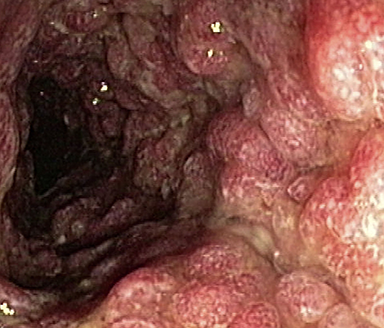
These photos are representative of colons diagnosed with varied severities of UC and not from patients taking SIMPONI®.
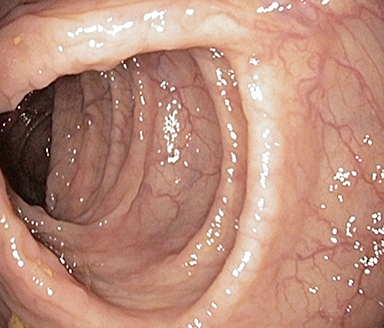
Healthy colon
Mild UC
Moderate UC
Severe UC
SIMPONI® is not for everyone. Only your doctor can decide if SIMPONI® is right for you.
Talk to your doctor to find out if SIMPONI® is right for you.

Talk to your doctor about UC symptom control and beginning to improve the appearance of the intestinal lining as possible treatment goals
As UC progresses, the appearance of the intestinal lining can become dry, brittle, and inflamed, and tiny sores can develop. Improving the appearance of the intestinal lining can be a realistic treatment goal in the management of UC. SIMPONI® is clinically proven to begin improving the appearance of the intestinal lining.
Meet Lindsey
Lindsey is a medical assistant from Georgia who works with UC patients being treated with SIMPONI®. She wants to share her experiences.
This is a modal window.
The Playback API request failed for an unknown reason
Interested in sharing your story?
Sign up

How does SIMPONI® work?

Before starting a new medication, it's important to know what it is, how it works, and what you can expect from treatment. Here's how SIMPONI® can help relieve the symptoms of moderately to severely active ulcerative colitis (UC).
The role of TNF-alpha

Tumor necrosis factor-alpha (TNF-alpha) is a protein made by your body's immune system. People with diseases such as UC have too much TNF-alpha. Excess amounts of TNF-alpha cause your immune system to mistakenly attack healthy cells in your gastrointestinal tract. This leads to inflammation, which causes the symptoms you experience with UC.
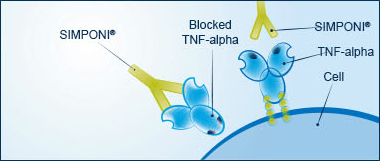
SIMPONI® targets, binds with, and blocks excess TNF-alpha, helping to control inflammation.
SIMPONI® targets TNF-alpha
SIMPONI® specifically targets excess TNF-alpha, helping to block an underlying cause of the symptoms of UC. SIMPONI® can bind with 2 forms of TNF-alpha, and at more than 1 location. It's important to know that blocking too much TNF-alpha can reduce your body's ability to fight infections.
Injectable UC treatment at a glance
Indication for Use
SIMPONI® is used for adults with moderately to severely active ulcerative colitis (UC) who are steroid dependent or have failed or were intolerant to conventional therapy.
SIMPONI®
(golimumab)
Humira®
(adalimumab)
Begin helping some of your symptoms

Get and keep your UC under control

Induce, achieve, and sustain remission

Induce and sustain remission
Begin to improve the appearance of your intestinal lining

SIMPONI®
(golimumab)

Humira®
(adalimumab)
SIMPONI®
(golimumab)

Induce, achieve, and sustain remission
Humira®
(adalimumab)

Induce and sustain remission
SIMPONI®
(golimumab)

Humira®
(adalimumab)
This chart is not intended to compare the safety, effectiveness, or uses of these treatments. Please talk with your doctor regarding each treatment.
SIMPONI® is a prescription medicine used to begin helping some of your symptoms, to begin improving the way the lining of your large intestine looks to your doctor during colonoscopy, and in people who respond to SIMPONI® to get their ulcerative colitis (UC) under control (induce remission) and keep it under control (sustain remission). SIMPONI® is used in adults with moderately to severely active UC when certain other UC medicines have not worked well enough or cannot be tolerated, or if it is necessary to continue taking steroid medicines.
Humira® is a prescription medicine used in adults to help get moderate to severe UC under control (induce remission) and keep it under control (sustain remission) when certain other medicines have not worked well enough. It is not known if Humira® is effective in people who stopped responding to or could not tolerate anti-TNF medicines. Humira® is a registered trademark of AbbVie Inc.
Treatment Frequency
Number of injections in the first year of treatment: SIMPONI® vs Humira®
Getting Started
SIMPONI®
(golimumab)
![]()
Day 1
Day 15
Humira®
(adalimumab)
![]()
Day 1
Day 15
Maintaining Treatment
1 injection every 4 weeks
SIMPONI®
(golimumab)

1 injection every 4 weeks
1 injection every 2 weeks
Humira®
(adalimumab)

1 injection every 2 weeks
This chart is not intended to compare the safety, effectiveness, or uses of these treatments. Please talk with your doctor regarding dosing.
Treatment with SIMPONI® begins with 3 starter injections: 2 injections on your first day of treatment, followed by 1 injection 2 weeks later. After these 3 starter injections, SIMPONI® requires just 1 injection every 4 weeks. If your doctor decides that you or your caregiver can give your injections at home, you will need to be trained on the proper way to self-inject SIMPONI® directly under the skin. Once you've learned how to self-inject, you can do it at home without visiting a doctor's office.
SIMPONI® is intended for use under the guidance and supervision of a physician. Patients may self-inject with SIMPONI® after physician approval and proper training.
Prior to initiating SIMPONI® and periodically during therapy, patients should be evaluated for active tuberculosis and tested for latent infection. Prior to initiating SIMPONI®, patients should be tested for hepatitis B viral infection.
While dosing frequency is important, there are other factors to consider.
Talk to your doctor about which treatment is right for you.
SIMPONI® (golimumab) Other Important Considerations
SIMPONI® is a medicine that affects your immune system. SIMPONI® can lower the ability of your immune system to fight infections. Some people have serious infections while taking SIMPONI®, including tuberculosis (TB), and infections caused by bacteria, fungi, or viruses that spread throughout their body. Some people have died from these serious infections.
- Your doctor should test you for TB and hepatitis B before starting SIMPONI®.
- Your doctor should monitor you closely for signs and symptoms of TB during treatment with SIMPONI®.
You should not start taking SIMPONI® if you have any kind of infection unless your doctor says it is okay.
To learn more about these and other risks, please read the Important Safety Information about SIMPONI® below, and the Medication Guide, and talk with your doctor.
WHAT IS SIMPONI® (golimumab)?
SIMPONI® is a prescription medicine used to treat adults with the medicine methotrexate to treat moderately to severely active rheumatoid arthritis (RA).
SIMPONI® is a prescription medicine used to treat adults with active psoriatic arthritis (PsA) alone or with methotrexate.
SIMPONI® is a prescription medicine used to treat adults with active ankylosing spondylitis (AS).
SIMPONI® is a prescription medicine used to treat adults and children weighing at least 33 pounds (15 kg) with moderately to severely active ulcerative colitis (UC).
IMPORTANT SAFETY INFORMATION
SERIOUS INFECTIONS
SIMPONI® (golimumab) is a prescription medicine. SIMPONI® can lower your ability to fight infections. There are reports of serious infections caused by bacteria, fungi, or viruses that have spread throughout the body, including tuberculosis (TB) and histoplasmosis. Some of these infections have been fatal. Your doctor will test you for TB before starting SIMPONI® and will monitor you for signs of TB during treatment. Tell your doctor if you have been in close contact with people with TB. Tell your doctor if you have been in a region (such as the Ohio and Mississippi River Valleys and the Southwest) where certain fungal infections like histoplasmosis or coccidioidomycosis are common.
You should not start SIMPONI® if you have any kind of infection. Tell your doctor if you are prone to or have a history of infections or have diabetes, HIV, or a weak immune system. You should also tell your doctor if you are currently being treated for an infection or if you have or develop any signs of an infection such as:
|
|
Your doctor will examine you for TB and perform a test to see if you have TB. If your doctor feels that you are at risk for TB, you may be treated with medicine for TB before you begin treatment with SIMPONI® and during treatment with SIMPONI®. Even if your TB test is negative, your doctor should carefully monitor you for TB infections while you are taking SIMPONI®. People who had a negative TB skin test before receiving SIMPONI® have developed active TB. Tell your doctor if you have any of the following symptoms while taking or after taking SIMPONI®:
|
|
CANCER
Unusual cancers have been reported in children and teenage patients taking TNF-blocker medicines. For children and adults taking TNF blockers, including SIMPONI®, the chances for getting lymphoma or other cancers may increase. Hepatosplenic T-cell lymphoma, a rare and fatal lymphoma, has occurred mostly in teenage or young adult males with Crohn’s disease or ulcerative colitis who were taking other TNF blockers with azathioprine or 6-mercaptopurine. You should tell your doctor if you have had or develop lymphoma or other cancers.
Some people treated with SIMPONI® have developed certain kinds of skin cancer. If any changes in the appearance of your skin or growths on your skin occur during or after your treatment with SIMPONI®, tell your doctor.
USE WITH OTHER DRUGS
Tell your doctor about all the medications you take including ORENCIA® (abatacept), KINERET® (anakinra), ACTEMRA® (tocilizumab), RITUXAN® (rituximab), or another TNF blocker, or if you are scheduled to or recently received a vaccine. People taking SIMPONI® should not receive live vaccines or treatment with a weakened bacteria (such as BCG for bladder cancer).
HEPATITIS B INFECTION
If you are a carrier of the hepatitis B virus (a virus that affects the liver), the virus can become active while you use SIMPONI®. Some of these cases have been fatal. Your doctor should do blood tests before and while you are using SIMPONI®. Tell your doctor if you know or think you may be a carrier of hepatitis B virus or if you experience signs of hepatitis B infection, such as:
|
|
HEART FAILURE
Heart failure, including new heart failure or worsening of heart failure that you already have, can occur or get worse in people who use TNF blockers, including SIMPONI®. If you develop new or worsening heart failure with SIMPONI®, you may need treatment in a hospital, and it may result in death. If you have heart failure before starting SIMPONI®, your condition should be watched closely during treatment with SIMPONI®. Call your doctor right away if you get new or worsening symptoms of heart failure like shortness of breath, swelling of your lower legs or feet, or sudden weight gain.
NERVOUS SYSTEM PROBLEMS
Rarely, people using TNF blockers, including SIMPONI®, can have nervous system problems such as multiple sclerosis or Guillain-Barré syndrome. Tell your doctor right away if you have symptoms like vision changes, weakness in your arms or legs, or numbness or tingling in any part of your body.
IMMUNE SYSTEM PROBLEMS
Rarely, people using TNF blockers have developed lupus-like symptoms. Tell your doctor if you have any symptoms such as a rash on your cheeks or other parts of the body, sensitivity to the sun, new joint or muscle pain, becoming very tired, chest pain or shortness of breath, or swelling of the feet, ankles, and/or legs.
LIVER PROBLEMS
Serious liver problems can happen in people using TNF blockers, including SIMPONI®. These problems can lead to liver failure and death. Call your doctor immediately if you develop symptoms such as feeling very tired, skin or eyes look yellow, poor appetite or vomiting, or pain on the right side of your stomach (abdomen).
BLOOD PROBLEMS
Low blood counts have been seen with SIMPONI®. If this occurs, your body may not make enough blood cells to help fight infections or help stop bleeding. Your doctor will check your blood counts before and during treatment. Tell your doctor if you have signs such as fever, bruising or bleeding easily, or looking pale.
ALLERGIC REACTIONS
Allergic reactions can happen in people who use TNF-blocker medicines, including SIMPONI®. Some reactions may be serious and can be life-threatening. Some of these reactions can happen after receiving your first dose of SIMPONI®. Stop using SIMPONI® and call your doctor right away if you have any symptoms of an allergic reaction such as hives, swollen face, breathing trouble, or chest pain.
OTHER CONSIDERATIONS TO TELL YOUR DOCTOR
Tell your doctor if you are allergic to rubber or latex. The needle cover on the prefilled syringe and SmartJect autoinjector contains dry natural rubber.
Tell your doctor if you are pregnant, planning to become pregnant, breastfeeding, plan to breastfeed, or have a baby and were using SIMPONI® during pregnancy. Tell your baby’s doctor before your baby receives any vaccine because of an increased risk of infection for up to 6 months after birth.
The most common side effects of SIMPONI® include: upper respiratory infection (runny nose, sore throat, and hoarseness or laryngitis), reaction at site of injection (redness, swelling, itching, pain, bruising, or tingling), and viral infections such as flu and oral cold sores.
PSORIASIS
New or worsening psoriasis symptoms may occur. Tell your doctor if you develop red scaly patches or raised bumps that are filled with pus. Your doctor may decide to stop your treatment with SIMPONI®.
These are not all of the possible side effects of SIMPONI®. Tell your doctor about any side effect that bothers you or does not go away. Call your doctor for medical advice about side effects.
Please read the full Prescribing Information and Medication Guide for SIMPONI® and discuss any questions you have with your doctor.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
cp-51591v5
WHAT IS SIMPONI® (golimumab)?
SIMPONI® is a prescription medicine used to treat adults with the medicine methotrexate to treat moderately to severely active rheumatoid arthritis (RA).
SIMPONI® is a prescription medicine used to treat adults with active psoriatic arthritis (PsA) alone or with methotrexate.
SIMPONI® is a prescription medicine used to treat adults with active ankylosing spondylitis (AS).
SIMPONI® is a prescription medicine used to treat adults and children weighing at least 33 pounds (15 kg) with moderately to severely active ulcerative colitis (UC).
IMPORTANT SAFETY INFORMATION
SERIOUS INFECTIONS
SIMPONI® (golimumab) is a prescription medicine. SIMPONI® can lower your ability to fight infections. There are reports of serious infections caused by bacteria, fungi, or viruses that have spread throughout the body, including tuberculosis (TB) and histoplasmosis. Some of these infections have been fatal. Your doctor will test you for TB before starting SIMPONI® and will monitor you for signs of TB during treatment. Tell your doctor if you have been in close contact with people with TB. Tell your doctor if you have been in a region (such as the Ohio and Mississippi River Valleys and the Southwest) where certain fungal infections like histoplasmosis or coccidioidomycosis are common.
You should not start SIMPONI® if you have any kind of infection. Tell your doctor if you are prone to or have a history of infections or have diabetes, HIV, or a weak immune system. You should also tell your doctor if you are currently being treated for an infection or if you have or develop any signs of an infection such as:
|
|
Your doctor will examine you for TB and perform a test to see if you have TB. If your doctor feels that you are at risk for TB, you may be treated with medicine for TB before you begin treatment with SIMPONI® and during treatment with SIMPONI®. Even if your TB test is negative, your doctor should carefully monitor you for TB infections while you are taking SIMPONI®. People who had a negative TB skin test before receiving SIMPONI® have developed active TB. Tell your doctor if you have any of the following symptoms while taking or after taking SIMPONI®:
|
|
CANCER
Unusual cancers have been reported in children and teenage patients taking TNF-blocker medicines. For children and adults taking TNF blockers, including SIMPONI®, the chances for getting lymphoma or other cancers may increase. Hepatosplenic T-cell lymphoma, a rare and fatal lymphoma, has occurred mostly in teenage or young adult males with Crohn’s disease or ulcerative colitis who were taking other TNF blockers with azathioprine or 6-mercaptopurine. You should tell your doctor if you have had or develop lymphoma or other cancers.
Some people treated with SIMPONI® have developed certain kinds of skin cancer. If any changes in the appearance of your skin or growths on your skin occur during or after your treatment with SIMPONI®, tell your doctor.
USE WITH OTHER DRUGS
Tell your doctor about all the medications you take including ORENCIA® (abatacept), KINERET® (anakinra), ACTEMRA® (tocilizumab), RITUXAN® (rituximab), or another TNF blocker, or if you are scheduled to or recently received a vaccine. People taking SIMPONI® should not receive live vaccines or treatment with a weakened bacteria (such as BCG for bladder cancer).
HEPATITIS B INFECTION
If you are a carrier of the hepatitis B virus (a virus that affects the liver), the virus can become active while you use SIMPONI®. Some of these cases have been fatal. Your doctor should do blood tests before and while you are using SIMPONI®. Tell your doctor if you know or think you may be a carrier of hepatitis B virus or if you experience signs of hepatitis B infection, such as:
|
|
HEART FAILURE
Heart failure, including new heart failure or worsening of heart failure that you already have, can occur or get worse in people who use TNF blockers, including SIMPONI®. If you develop new or worsening heart failure with SIMPONI®, you may need treatment in a hospital, and it may result in death. If you have heart failure before starting SIMPONI®, your condition should be watched closely during treatment with SIMPONI®. Call your doctor right away if you get new or worsening symptoms of heart failure like shortness of breath, swelling of your lower legs or feet, or sudden weight gain.
NERVOUS SYSTEM PROBLEMS
Rarely, people using TNF blockers, including SIMPONI®, can have nervous system problems such as multiple sclerosis or Guillain-Barré syndrome. Tell your doctor right away if you have symptoms like vision changes, weakness in your arms or legs, or numbness or tingling in any part of your body.
IMMUNE SYSTEM PROBLEMS
Rarely, people using TNF blockers have developed lupus-like symptoms. Tell your doctor if you have any symptoms such as a rash on your cheeks or other parts of the body, sensitivity to the sun, new joint or muscle pain, becoming very tired, chest pain or shortness of breath, or swelling of the feet, ankles, and/or legs.
LIVER PROBLEMS
Serious liver problems can happen in people using TNF blockers, including SIMPONI®. These problems can lead to liver failure and death. Call your doctor immediately if you develop symptoms such as feeling very tired, skin or eyes look yellow, poor appetite or vomiting, or pain on the right side of your stomach (abdomen).
BLOOD PROBLEMS
Low blood counts have been seen with SIMPONI®. If this occurs, your body may not make enough blood cells to help fight infections or help stop bleeding. Your doctor will check your blood counts before and during treatment. Tell your doctor if you have signs such as fever, bruising or bleeding easily, or looking pale.
ALLERGIC REACTIONS
Allergic reactions can happen in people who use TNF-blocker medicines, including SIMPONI®. Some reactions may be serious and can be life-threatening. Some of these reactions can happen after receiving your first dose of SIMPONI®. Stop using SIMPONI® and call your doctor right away if you have any symptoms of an allergic reaction such as hives, swollen face, breathing trouble, or chest pain.
OTHER CONSIDERATIONS TO TELL YOUR DOCTOR
Tell your doctor if you are allergic to rubber or latex. The needle cover on the prefilled syringe and SmartJect autoinjector contains dry natural rubber.
Tell your doctor if you are pregnant, planning to become pregnant, breastfeeding, plan to breastfeed, or have a baby and were using SIMPONI® during pregnancy. Tell your baby’s doctor before your baby receives any vaccine because of an increased risk of infection for up to 6 months after birth.
The most common side effects of SIMPONI® include: upper respiratory infection (runny nose, sore throat, and hoarseness or laryngitis), reaction at site of injection (redness, swelling, itching, pain, bruising, or tingling), and viral infections such as flu and oral cold sores.
PSORIASIS
New or worsening psoriasis symptoms may occur. Tell your doctor if you develop red scaly patches or raised bumps that are filled with pus. Your doctor may decide to stop your treatment with SIMPONI®.
These are not all of the possible side effects of SIMPONI®. Tell your doctor about any side effect that bothers you or does not go away. Call your doctor for medical advice about side effects.
Please read the full Prescribing Information and Medication Guide for SIMPONI® and discuss any questions you have with your doctor.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
cp-51591v5
WHAT IS SIMPONI® (golimumab)?
SIMPONI® is a prescription medicine used to treat adults with the medicine methotrexate to treat moderately to severely active rheumatoid arthritis (RA).
SIMPONI® is a prescription medicine used to treat adults with active psoriatic arthritis (PsA) alone or with methotrexate.
SIMPONI® is a prescription medicine used to treat adults with active ankylosing spondylitis (AS).
SIMPONI® is a prescription medicine used to treat adults and children weighing at least 33 pounds (15 kg) with moderately to severely active ulcerative colitis (UC).
IMPORTANT SAFETY INFORMATION
SERIOUS INFECTIONS
SIMPONI® (golimumab) is a prescription medicine. SIMPONI® can lower your ability to fight infections. There are reports of serious infections caused by bacteria, fungi, or viruses that have spread throughout the body, including tuberculosis (TB) and histoplasmosis. Some of these infections have been fatal. Your doctor will test you for TB before starting SIMPONI® and will monitor you for signs of TB during treatment. Tell your doctor if you have been in close contact with people with TB. Tell your doctor if you have been in a region (such as the Ohio and Mississippi River Valleys and the Southwest) where certain fungal infections like histoplasmosis or coccidioidomycosis are common.
You should not start SIMPONI® if you have any kind of infection. Tell your doctor if you are prone to or have a history of infections or have diabetes, HIV, or a weak immune system. You should also tell your doctor if you are currently being treated for an infection or if you have or develop any signs of an infection such as:
|
|
Your doctor will examine you for TB and perform a test to see if you have TB. If your doctor feels that you are at risk for TB, you may be treated with medicine for TB before you begin treatment with SIMPONI® and during treatment with SIMPONI®. Even if your TB test is negative, your doctor should carefully monitor you for TB infections while you are taking SIMPONI®. People who had a negative TB skin test before receiving SIMPONI® have developed active TB. Tell your doctor if you have any of the following symptoms while taking or after taking SIMPONI®:
|
|
CANCER
Unusual cancers have been reported in children and teenage patients taking TNF-blocker medicines. For children and adults taking TNF blockers, including SIMPONI®, the chances for getting lymphoma or other cancers may increase. Hepatosplenic T-cell lymphoma, a rare and fatal lymphoma, has occurred mostly in teenage or young adult males with Crohn’s disease or ulcerative colitis who were taking other TNF blockers with azathioprine or 6-mercaptopurine. You should tell your doctor if you have had or develop lymphoma or other cancers.
Some people treated with SIMPONI® have developed certain kinds of skin cancer. If any changes in the appearance of your skin or growths on your skin occur during or after your treatment with SIMPONI®, tell your doctor.
USE WITH OTHER DRUGS
Tell your doctor about all the medications you take including ORENCIA® (abatacept), KINERET® (anakinra), ACTEMRA® (tocilizumab), RITUXAN® (rituximab), or another TNF blocker, or if you are scheduled to or recently received a vaccine. People taking SIMPONI® should not receive live vaccines or treatment with a weakened bacteria (such as BCG for bladder cancer).
HEPATITIS B INFECTION
If you are a carrier of the hepatitis B virus (a virus that affects the liver), the virus can become active while you use SIMPONI®. Some of these cases have been fatal. Your doctor should do blood tests before and while you are using SIMPONI®. Tell your doctor if you know or think you may be a carrier of hepatitis B virus or if you experience signs of hepatitis B infection, such as:
|
|
HEART FAILURE
Heart failure, including new heart failure or worsening of heart failure that you already have, can occur or get worse in people who use TNF blockers, including SIMPONI®. If you develop new or worsening heart failure with SIMPONI®, you may need treatment in a hospital, and it may result in death. If you have heart failure before starting SIMPONI®, your condition should be watched closely during treatment with SIMPONI®. Call your doctor right away if you get new or worsening symptoms of heart failure like shortness of breath, swelling of your lower legs or feet, or sudden weight gain.
NERVOUS SYSTEM PROBLEMS
Rarely, people using TNF blockers, including SIMPONI®, can have nervous system problems such as multiple sclerosis or Guillain-Barré syndrome. Tell your doctor right away if you have symptoms like vision changes, weakness in your arms or legs, or numbness or tingling in any part of your body.
IMMUNE SYSTEM PROBLEMS
Rarely, people using TNF blockers have developed lupus-like symptoms. Tell your doctor if you have any symptoms such as a rash on your cheeks or other parts of the body, sensitivity to the sun, new joint or muscle pain, becoming very tired, chest pain or shortness of breath, or swelling of the feet, ankles, and/or legs.
LIVER PROBLEMS
Serious liver problems can happen in people using TNF blockers, including SIMPONI®. These problems can lead to liver failure and death. Call your doctor immediately if you develop symptoms such as feeling very tired, skin or eyes look yellow, poor appetite or vomiting, or pain on the right side of your stomach (abdomen).
BLOOD PROBLEMS
Low blood counts have been seen with SIMPONI®. If this occurs, your body may not make enough blood cells to help fight infections or help stop bleeding. Your doctor will check your blood counts before and during treatment. Tell your doctor if you have signs such as fever, bruising or bleeding easily, or looking pale.
ALLERGIC REACTIONS
Allergic reactions can happen in people who use TNF-blocker medicines, including SIMPONI®. Some reactions may be serious and can be life-threatening. Some of these reactions can happen after receiving your first dose of SIMPONI®. Stop using SIMPONI® and call your doctor right away if you have any symptoms of an allergic reaction such as hives, swollen face, breathing trouble, or chest pain.
OTHER CONSIDERATIONS TO TELL YOUR DOCTOR
Tell your doctor if you are allergic to rubber or latex. The needle cover on the prefilled syringe and SmartJect autoinjector contains dry natural rubber.
Tell your doctor if you are pregnant, planning to become pregnant, breastfeeding, plan to breastfeed, or have a baby and were using SIMPONI® during pregnancy. Tell your baby’s doctor before your baby receives any vaccine because of an increased risk of infection for up to 6 months after birth.
The most common side effects of SIMPONI® include: upper respiratory infection (runny nose, sore throat, and hoarseness or laryngitis), reaction at site of injection (redness, swelling, itching, pain, bruising, or tingling), and viral infections such as flu and oral cold sores.
PSORIASIS
New or worsening psoriasis symptoms may occur. Tell your doctor if you develop red scaly patches or raised bumps that are filled with pus. Your doctor may decide to stop your treatment with SIMPONI®.
These are not all of the possible side effects of SIMPONI®. Tell your doctor about any side effect that bothers you or does not go away. Call your doctor for medical advice about side effects.
Please read the full Prescribing Information and Medication Guide for SIMPONI® and discuss any questions you have with your doctor.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
cp-51591v5
SIMPONI® is a prescription medicine used to treat adults with the medicine methotrexate to treat moderately to severely active rheumatoid arthritis (RA).
SIMPONI® is a prescription medicine used to treat adults with active psoriatic arthritis (PsA) alone or with methotrexate.
SIMPONI® is a prescription medicine used to treat adults with active ankylosing spondylitis (AS).
SIMPONI® is a prescription medicine used to treat adults and children weighing at least 33 pounds (15 kg) with moderately to severely active ulcerative colitis (UC).
SERIOUS INFECTIONS
SIMPONI® (golimumab) is a prescription medicine. SIMPONI® can lower your ability to fight infections. There are reports of serious infections caused by bacteria, fungi, or viruses that have spread throughout the body, including tuberculosis (TB) and histoplasmosis. Some of these infections have been fatal. Your doctor will test you for TB before starting SIMPONI® and will monitor you for signs of TB during treatment. Tell your doctor if you have been in close contact with people with TB. Tell your doctor if you have been in a region (such as the Ohio and Mississippi River Valleys and the Southwest) where certain fungal infections like histoplasmosis or coccidioidomycosis are common.
You should not start SIMPONI® if you have any kind of infection. Tell your doctor if you are prone to or have a history of infections or have diabetes, HIV, or a weak immune system. You should also tell your doctor if you are currently being treated for an infection or if you have or develop any signs of an infection such as:
|
|
Your doctor will examine you for TB and perform a test to see if you have TB. If your doctor feels that you are at risk for TB, you may be treated with medicine for TB before you begin treatment with SIMPONI® and during treatment with SIMPONI®. Even if your TB test is negative, your doctor should carefully monitor you for TB infections while you are taking SIMPONI®. People who had a negative TB skin test before receiving SIMPONI® have developed active TB. Tell your doctor if you have any of the following symptoms while taking or after taking SIMPONI®:
|
|
CANCER
Unusual cancers have been reported in children and teenage patients taking TNF-blocker medicines. For children and adults taking TNF blockers, including SIMPONI®, the chances for getting lymphoma or other cancers may increase. Hepatosplenic T-cell lymphoma, a rare and fatal lymphoma, has occurred mostly in teenage or young adult males with Crohn’s disease or ulcerative colitis who were taking other TNF blockers with azathioprine or 6-mercaptopurine. You should tell your doctor if you have had or develop lymphoma or other cancers.
Some people treated with SIMPONI® have developed certain kinds of skin cancer. If any changes in the appearance of your skin or growths on your skin occur during or after your treatment with SIMPONI®, tell your doctor.
USE WITH OTHER DRUGS
Tell your doctor about all the medications you take including ORENCIA® (abatacept), KINERET® (anakinra), ACTEMRA® (tocilizumab), RITUXAN® (rituximab), or another TNF blocker, or if you are scheduled to or recently received a vaccine. People taking SIMPONI® should not receive live vaccines or treatment with a weakened bacteria (such as BCG for bladder cancer).
HEPATITIS B INFECTION
If you are a carrier of the hepatitis B virus (a virus that affects the liver), the virus can become active while you use SIMPONI®. Some of these cases have been fatal. Your doctor should do blood tests before and while you are using SIMPONI®. Tell your doctor if you know or think you may be a carrier of hepatitis B virus or if you experience signs of hepatitis B infection, such as:
|
|
HEART FAILURE
Heart failure, including new heart failure or worsening of heart failure that you already have, can occur or get worse in people who use TNF blockers, including SIMPONI®. If you develop new or worsening heart failure with SIMPONI®, you may need treatment in a hospital, and it may result in death. If you have heart failure before starting SIMPONI®, your condition should be watched closely during treatment with SIMPONI®. Call your doctor right away if you get new or worsening symptoms of heart failure like shortness of breath, swelling of your lower legs or feet, or sudden weight gain.
NERVOUS SYSTEM PROBLEMS
Rarely, people using TNF blockers, including SIMPONI®, can have nervous system problems such as multiple sclerosis or Guillain-Barré syndrome. Tell your doctor right away if you have symptoms like vision changes, weakness in your arms or legs, or numbness or tingling in any part of your body.
IMMUNE SYSTEM PROBLEMS
Rarely, people using TNF blockers have developed lupus-like symptoms. Tell your doctor if you have any symptoms such as a rash on your cheeks or other parts of the body, sensitivity to the sun, new joint or muscle pain, becoming very tired, chest pain or shortness of breath, or swelling of the feet, ankles, and/or legs.
LIVER PROBLEMS
Serious liver problems can happen in people using TNF blockers, including SIMPONI®. These problems can lead to liver failure and death. Call your doctor immediately if you develop symptoms such as feeling very tired, skin or eyes look yellow, poor appetite or vomiting, or pain on the right side of your stomach (abdomen).
BLOOD PROBLEMS
Low blood counts have been seen with SIMPONI®. If this occurs, your body may not make enough blood cells to help fight infections or help stop bleeding. Your doctor will check your blood counts before and during treatment. Tell your doctor if you have signs such as fever, bruising or bleeding easily, or looking pale.
ALLERGIC REACTIONS
Allergic reactions can happen in people who use TNF-blocker medicines, including SIMPONI®. Some reactions may be serious and can be life-threatening. Some of these reactions can happen after receiving your first dose of SIMPONI®. Stop using SIMPONI® and call your doctor right away if you have any symptoms of an allergic reaction such as hives, swollen face, breathing trouble, or chest pain.
OTHER CONSIDERATIONS TO TELL YOUR DOCTOR
Tell your doctor if you are allergic to rubber or latex. The needle cover on the prefilled syringe and SmartJect autoinjector contains dry natural rubber.
Tell your doctor if you are pregnant, planning to become pregnant, breastfeeding, plan to breastfeed, or have a baby and were using SIMPONI® during pregnancy. Tell your baby’s doctor before your baby receives any vaccine because of an increased risk of infection for up to 6 months after birth.
The most common side effects of SIMPONI® include: upper respiratory infection (runny nose, sore throat, and hoarseness or laryngitis), reaction at site of injection (redness, swelling, itching, pain, bruising, or tingling), and viral infections such as flu and oral cold sores.
PSORIASIS
New or worsening psoriasis symptoms may occur. Tell your doctor if you develop red scaly patches or raised bumps that are filled with pus. Your doctor may decide to stop your treatment with SIMPONI®.
These are not all of the possible side effects of SIMPONI®. Tell your doctor about any side effect that bothers you or does not go away. Call your doctor for medical advice about side effects.
Please read the full Prescribing Information and Medication Guide for SIMPONI® and discuss any questions you have with your doctor.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
cp-51591v5
