Ulcerative Colitis Treatment With SIMPONI®
While individual results may vary, SIMPONI® was proven in clinical studies to:
- Help people with moderately to severely active UC improve their symptoms in as few as 6 weeks
- Maintain improvement of symptoms through 54 weeks with a single injection once every 4 weeks for those people who responded to SIMPONI®
- Begin to improve the appearance of the intestinal lining in as few as 6 weeks
AT 6 WEEKS
In an induction study, about 5 out of 10 patients receiving 200 mg/100 mg SIMPONI® had improvement in their UC symptoms.
SIMPONI® 200 mg/100 mg* (N=253)
VS
Placebo (N=251)
Induction study primary endpoint
*
In the induction study, patients received SIMPONI® 200 mg subcutaneously (SC)
(under the skin) at
Week 0 and 100 mg SC at Week 2.
AT 54 WEEKS
After 1 year of treatment, 5 out of 10 patients receiving SIMPONI® 100 mg maintained this improvement in their UC symptoms.
SIMPONI® 100 mg (N=151)
VS
Placebo (N=154)
Maintenance study primary endpoint in patients who responded in the induction study
AT 6 WEEKS
In an induction study, more than 4 out of 10 patients receiving 200 mg/100 mg SIMPONI® experienced improvement of endoscopic appearance of the mucosa.
SIMPONI® 200 mg/100 mg* (N=253)
VS
Placebo (N=251)
Induction study major secondary endpoint
How Does SIMPONI® Work?
Before starting a new medication, it's important to know what it is and how it works. Here's a closer look at SIMPONI®.
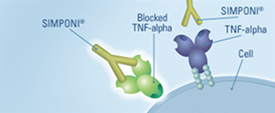
SIMPONI® is a TNF blocker biologic medicine
It targets and blocks a protein called TNF-alpha (tumor necrosis factor-alpha)
- TNF-alpha is made by your immune system
- People with diseases such as UC have too much TNF-alpha
- Excess amounts of TNF-alpha cause your immune system to mistakenly attack healthy cells in their gastrointestinal tract
- This can lead to inflammation, which causes the symptoms that you experience with UC
SIMPONI® specifically targets excess TNF-alpha, helping to block an underlying cause of the symptoms of UC. SIMPONI® can bind with 2 forms of TNF-alpha, and at more than 1 location.
SIMPONI® targets, binds with, and blocks excess TNF-alpha, helping to control inflammation.
SIMPONI® targets, binds with, and blocks excess TNF-alpha, helping to control inflammation
Blocking too much TNF-alpha can make it harder for your body to fight infections. See your doctor regularly. Tell him or her if you have side effects.
SIMPONI® is not for everyone. Only your doctor can decide if SIMPONI® is right for you.